Enantioselective synthesis of natural product analogues with quaternary carbon centres
Research group:
Synthesis & Bioactivity
Leader of the research group and project supervisor:
Dr. Eliška Matoušová, eliska.matousova@natur.cuni.cz
Department:
Department of Organic Chemistry
Web pages of the research group:
http://orgchem.natur.cuni.cz/matousova/
Project summary:
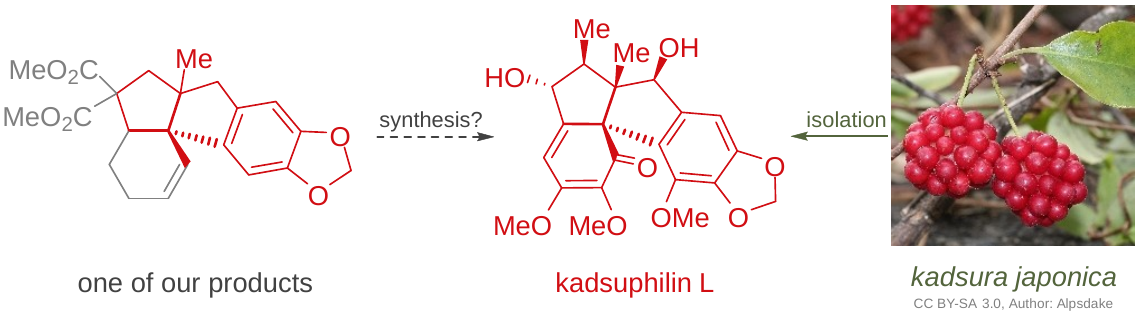
All-carbon quaternary centres occur widely in natural products, as well as pharmaceutically important compounds. Their enantioselective synthesis, however, still represents one of the greatest challenges in synthetic organic chemistry. This PhD project will continue in an ongoing research of the group dealing with the quaternary carbon centres. We previously developed a method for an enantioselective synthesis of compounds structurally resembling Amaryllidaceae alkaloids by using tandem cyclisation/Suzuki cross-coupling and subsequent halocarbocyclisation as the key reaction sequence. In this project, on the other hand, it is planned to employ palladium-catalysed oxidative coupling or Heck reaction to form such quaternary centres, using sligthly modified starting materials. By that means the synthesis of compounds resembling natural lignans kadsuphilins L and M should be achieved. Lignans of Schisandraceae family of climbing plants, where kadsuphilins belong, were found to posess various pharmacological activities, including antitumor, anti-HIV, antihepatitis, antineurodegenerative and antioxidative effects. Specifically, kadsuphilins L and M were isolated from Kadsura plants and their total synthesis has not been reported so far. Hence, it would be valuable to prepare these natural products and/or their derivatives and evaluate their biological properties.
Recent publications of the project supervisor:
1. Mikušek, J.; Jansa, P.; Jagtap, P. R.; Vašíček, T.; Císařová, I.; Matoušová, E. Chem. – Eur. J. 2018, 24, 10069.
2. Matoušová, E.; Koukal, P.; Formánek, B.; Kotora, M. Org. Lett. 2016, 18, 5656.
3. Matoušová, E.; Gyepes, R.; Císařová, I.; Kotora, M. Adv. Synth. Catal. 2016, 358, 254.
4. Schwartz, B. D.; Matoušová, E.; White, R.; Banwell, M. G.; Willis, A. C. Org. Lett. 2013, 15, 1934.
5. Matoušová, E.; Růžička, A.; Kuneš, J.; Králová, J.; Pour, M. Chem. Commun. 2011, 47, 9390.
Current grant support of the group: PRIMUS/17/SCI/14
Deadline is closed